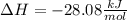Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Calculate Δ H o for the reaction. CH3OH + HCl → CH3Cl + H2O answer is in kJ/mol ....
Questions

History, 09.02.2021 23:20

Mathematics, 09.02.2021 23:20

Mathematics, 09.02.2021 23:20

History, 09.02.2021 23:20

English, 09.02.2021 23:20


Mathematics, 09.02.2021 23:20

Mathematics, 09.02.2021 23:20

History, 09.02.2021 23:20


Mathematics, 09.02.2021 23:20



English, 09.02.2021 23:20

Computers and Technology, 09.02.2021 23:20


Mathematics, 09.02.2021 23:20

Spanish, 09.02.2021 23:20

Mathematics, 09.02.2021 23:20

Mathematics, 09.02.2021 23:20