
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If the reaction starts with 2.15 mol of hydrogen bromide in 1.0 liter, and decomposes to 36.7%, what is the equilibrium constant of the decomposition of hydrogen bromide

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

You know the right answer?
Hydrogen bromide decomposes when heated to 437C according to the equation: 2HBr(g) H2(g) Br2(g). If...
Questions

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Social Studies, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

World Languages, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

Mathematics, 10.09.2020 05:01

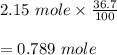
![K_c=\frac{[H_2][Br_2]}{[HBr]^2} \\\\=\frac{(0.395)(0.395)}{(1.361)^2} \\\\=\frac{0.156025}{1.852321} \\\\=0.084](/tpl/images/0685/5664/e5d29.png)


