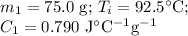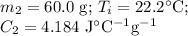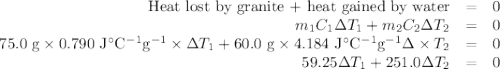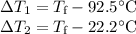Chemistry, 12.06.2020 21:57 dinosaur10
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC. Determine the final temperature (in oC) when they reach thermal equilibrium. Assume no heat loss to the surroundings. Specific heats: granite = 0.790 J/(goC), water = 4.184 J/goC

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC....
Questions







Biology, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20

Physics, 13.04.2021 19:20

Chemistry, 13.04.2021 19:20


Mathematics, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20



English, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20