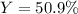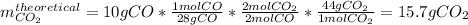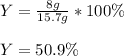Chemistry, 13.06.2020 05:57 michaelwarren8728
Consider the reaction 2 CO + O2 → 2 CO2 .What is the percent yield of carbon dioxide
(MW = 44 g/mol) if the reaction of 10 g of carbon monoxide (MW = 28 g/mol) with
excess O2 produces 8 g of carbon dioxide? Answer in units of %.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 10:30
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
You know the right answer?
Consider the reaction 2 CO + O2 → 2 CO2 .What is the percent yield of carbon dioxide
(MW = 44 g/mol...
Questions





English, 30.06.2021 04:00

Mathematics, 30.06.2021 04:00

Mathematics, 30.06.2021 04:00

History, 30.06.2021 04:00





Mathematics, 30.06.2021 04:00


English, 30.06.2021 04:00


Advanced Placement (AP), 30.06.2021 04:00


Mathematics, 30.06.2021 04:00

Arts, 30.06.2021 04:00