

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2


Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
Determine the temperature of 2.49mol of a gas contained in a 1.00L vessel at a pressure of 1.41atm....
Questions

Biology, 05.05.2020 13:27



Advanced Placement (AP), 05.05.2020 13:27


Mathematics, 05.05.2020 13:27

Mathematics, 05.05.2020 13:27

History, 05.05.2020 13:27


Engineering, 05.05.2020 13:27

History, 05.05.2020 13:27









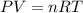
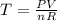
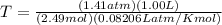
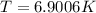
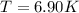 .
.

