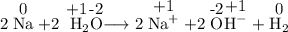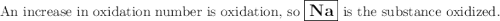Given the reaction: 2na + 2h2o → 2na+ + 2oh− + h2
which substance is oxidized?
1.
...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Questions



Mathematics, 04.05.2021 18:10

Mathematics, 04.05.2021 18:10

Mathematics, 04.05.2021 18:10





Mathematics, 04.05.2021 18:10


Mathematics, 04.05.2021 18:10

Geography, 04.05.2021 18:10




Mathematics, 04.05.2021 18:10


Mathematics, 04.05.2021 18:10