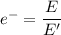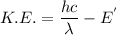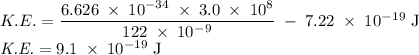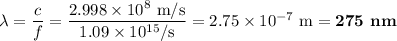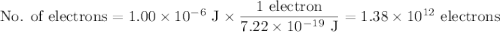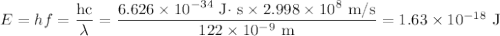Chemistry, 12.06.2020 05:57 coryowens44
Molybdenum metal requires a photon with a minimum frequency of 1.09x1015s-1before it can emit an electron via the photoelectric effect.
a) What is the minimum energy needed to eject an electron?
b)What wavelength of radiation (in nm) will provide a photon of this energy?
c)How many electrons can be freed by a burst of radiation whose total energy is 1.00 μJ, assuming one photon causes one electron to be freed? (μ= micro = 10-6)
d) If molybdenum is irradiated with light of 122nm, what is the maximum kinetic energy of the emitted electrons?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Molybdenum metal requires a photon with a minimum frequency of 1.09x1015s-1before it can emit an ele...
Questions

Biology, 11.10.2020 06:01


English, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01

Computers and Technology, 11.10.2020 06:01


Mathematics, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01



Mathematics, 11.10.2020 06:01


Health, 11.10.2020 06:01

History, 11.10.2020 06:01


Physics, 11.10.2020 06:01


Biology, 11.10.2020 06:01


Mathematics, 11.10.2020 06:01

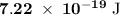 .
.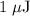 energy has been
energy has been 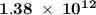 .
.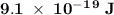 .
.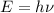
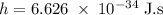
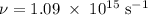
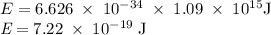
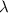 ) of the radiation has been given by:
) of the radiation has been given by:
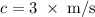
 .
.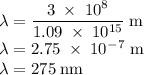
 ) burst out can be given as:
) burst out can be given as: