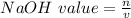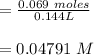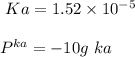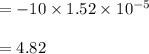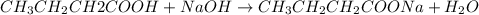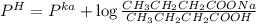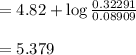Chemistry, 11.06.2020 17:57 lexiecooley
A 1.44 L buffer solution consists of 0.137 M butanoic acid and 0.275 M sodium butanoate. Calculate the pH of the solution following the addition of 0.069 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The Ka of butanoic acid is 1.52×10−5 .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

You know the right answer?
A 1.44 L buffer solution consists of 0.137 M butanoic acid and 0.275 M sodium butanoate. Calculate t...
Questions




Mathematics, 07.04.2020 01:30





Mathematics, 07.04.2020 01:30

English, 07.04.2020 01:30

English, 07.04.2020 01:30

Mathematics, 07.04.2020 01:30





Health, 07.04.2020 01:30


Mathematics, 07.04.2020 01:30

English, 07.04.2020 01:30

 ".
".