Chemistry, 12.06.2020 02:57 lilinicholeb
From the unbalanced reaction: B2H6 + O2 ---> HBO2 + H2O
How many grams of O2 (32g/mol) will be needed to burn 36.1 g of B2H6 (Molar mass = 27.67g/mol)? g
Include the correct number of significant figures in your final answer

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
From the unbalanced reaction: B2H6 + O2 ---> HBO2 + H2O
How many grams of O2 (32g/mol) will be n...
Questions

Mathematics, 21.08.2019 23:50

History, 21.08.2019 23:50














English, 21.08.2019 23:50

Mathematics, 21.08.2019 23:50

Mathematics, 21.08.2019 23:50

Mathematics, 21.08.2019 23:50

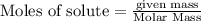
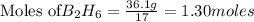
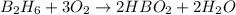
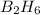 require = 3 moles of
require = 3 moles of 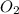
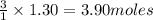 of
of