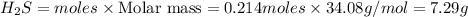Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 18:00
Amolecule is a(n) - a. element that physically combines with another element. b. particle composed of two or more atoms bonded together covalently. c. element that isn't bonded to another element.
Answers: 1

Chemistry, 23.06.2019 22:30
What is the thermodynamic driving force for dissolving a solid in a liquid if it is an endothermic process (which reduces the entropy of the surroundings)? 1. the combustion of propane. 2. the increase of the entropy of the system. 3. increased temperature of the system and surroundings. 4. the decrease of the entropy of the system. 5. the increase or decrease of the entropy of the system?
Answers: 1

You know the right answer?
Using the following balanced chemical equation 8 H2 + S8à 8 H2S. Determine the mass of the product (...
Questions


Mathematics, 29.11.2021 23:50

English, 29.11.2021 23:50


Mathematics, 29.11.2021 23:50

Mathematics, 29.11.2021 23:50

SAT, 29.11.2021 23:50







SAT, 29.11.2021 23:50

Mathematics, 29.11.2021 23:50


SAT, 29.11.2021 23:50

English, 29.11.2021 23:50

Mathematics, 29.11.2021 23:50

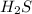 will be produced from the given masses of both reactants.
will be produced from the given masses of both reactants.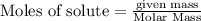
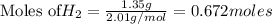
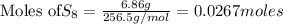
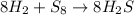
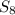 require = 8 moles of
require = 8 moles of 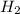
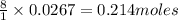 of
of