
Chemistry, 12.06.2020 02:57 eddsworldfrantic
P, Q and R react in aqueous solution according to the following equation:
P + 50 + 6R → 3T + 3U
The kinetics of the above reaction was studied by the method of initial rates and the
experimental results obtained are shown in the table below
MCQ question (reaction kinetics)
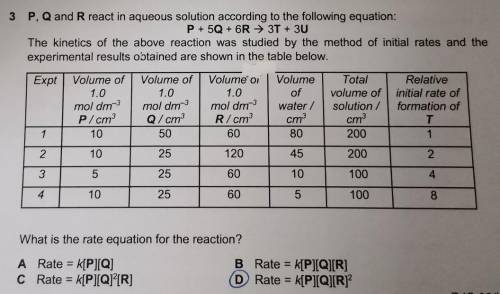

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement justifies that phosphine (ph3) is a polar molecule?
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
P, Q and R react in aqueous solution according to the following equation:
P + 50 + 6R → 3T + 3U
Questions

Mathematics, 29.12.2019 18:31









Mathematics, 29.12.2019 18:31

Chemistry, 29.12.2019 18:31

Chemistry, 29.12.2019 18:31

Mathematics, 29.12.2019 18:31


Health, 29.12.2019 18:31


Mathematics, 29.12.2019 18:31





