
Aqueous sulfuric acid H2SO4 will react with solid sodium hydroxide NaOH to produce aqueous sodium sulfate Na2SO4 and liquid water H2O. Suppose 62. g of sulfuric acid is mixed with 33.8 g of sodium hydroxide. Calculate the minimum mass of sulfuric acid that could be left over by the chemical reaction. Round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1


Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

You know the right answer?
Aqueous sulfuric acid H2SO4 will react with solid sodium hydroxide NaOH to produce aqueous sodium su...
Questions


Mathematics, 08.09.2021 23:00

History, 08.09.2021 23:00

Physics, 08.09.2021 23:00

History, 08.09.2021 23:00

Mathematics, 08.09.2021 23:00




Mathematics, 08.09.2021 23:00

Mathematics, 08.09.2021 23:00

Chemistry, 08.09.2021 23:00


Chemistry, 08.09.2021 23:00





Mathematics, 08.09.2021 23:00

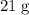 .
.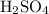 (a diprotic acid) reacts with
(a diprotic acid) reacts with 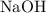 (a monoprotic base) at a one-to-two ratio:
(a monoprotic base) at a one-to-two ratio: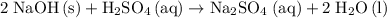 .
.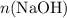 and
and 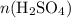 represent the number of moles of the two compounds reacted, then:
represent the number of moles of the two compounds reacted, then: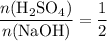 .
.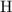 :
: 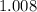 .
.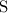 :
: 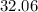 .
. :
: 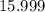 .
.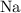 :
: 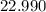 .
.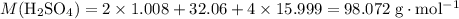 .
.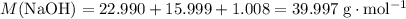 .
.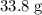 of
of 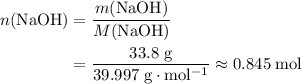 .
.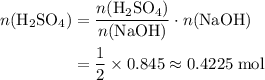 .
.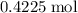 of
of 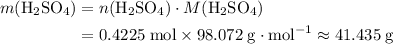 .
.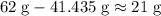 (rounded to two significant figures.)
(rounded to two significant figures.)

