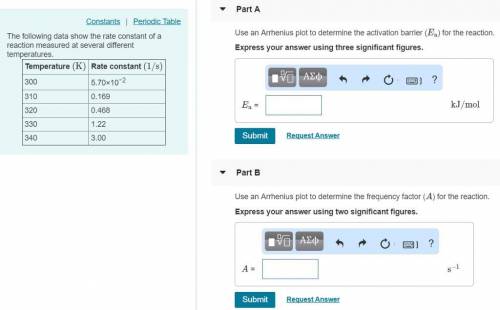
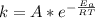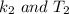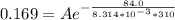The following data show the rate constant of a reaction measured at several different temperatures. Temperature (K) Rate Constant (1/s) 310 0.194 320 0.554 330 1.48 340 3.74 350 8.97 Part APart complete Use an Arrhenius plot to determine the activation barrier for the reaction. Express your answer using three significant figures. Ea

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 15:00
Charlene puts together two isosceles triangles so that they share a base, creating a kite. the legs of the triangles are 10 inches and 17 inches, respectively. if the length of the base for both triangles is 16 inches long, what is the length of the kite’s other diagonal? 6 inches inches inches 21 inchesanswer is d on e2020edit: it's geometry not chemistry, sorry.
Answers: 3
You know the right answer?
The following data show the rate constant of a reaction measured at several different temperatures....
Questions


Advanced Placement (AP), 29.07.2019 00:00

Geography, 29.07.2019 00:00



Mathematics, 29.07.2019 00:00

Advanced Placement (AP), 29.07.2019 00:00


Mathematics, 29.07.2019 00:00

Mathematics, 29.07.2019 00:00

Mathematics, 29.07.2019 00:00



Chemistry, 29.07.2019 00:00



Mathematics, 29.07.2019 00:00

Chemistry, 29.07.2019 00:00

Social Studies, 29.07.2019 00:00

Mathematics, 29.07.2019 00:00

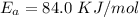

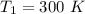
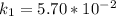
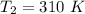
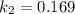
![ln [\frac{k_2}{k_1} ] = \frac{E_a}{R} [\frac{1}{T_1} - \frac{1}{T_2} ]](/tpl/images/0683/3945/2ceab.png)
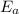 is the activation barrier for the reaction
is the activation barrier for the reaction 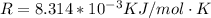
![ln [\frac{0.169}{6*10^-2{}} ] = \frac{E_a}{8.314*10^{-3}} [\frac{1}{300} - \frac{1}{310} ]](/tpl/images/0683/3945/6f5a8.png)