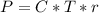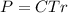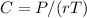Chemistry, 11.06.2020 16:02 bethdove9466
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant . Suppose the osmotic pressure of a certain solution is measured to be at an absolute temperature o of 312. K. Write an equation that will let you calculate the molarity c of this solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
An example of technology is the a. addition of a side group to an organic molecule during synthesis. b. use of a new antibiotic to fight an infection. c. measurement of iron concentration in a water sample. d. study of atomic fusion reactions
Answers: 3

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1
You know the right answer?
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute tempe...
Questions

Mathematics, 17.02.2021 09:40

Advanced Placement (AP), 17.02.2021 09:40

Computers and Technology, 17.02.2021 09:40

Chemistry, 17.02.2021 09:40

Mathematics, 17.02.2021 09:40

English, 17.02.2021 09:40

Mathematics, 17.02.2021 09:40

Spanish, 17.02.2021 09:40

History, 17.02.2021 09:40


Mathematics, 17.02.2021 09:40


Mathematics, 17.02.2021 09:50


Physics, 17.02.2021 09:50



Mathematics, 17.02.2021 09:50


Mathematics, 17.02.2021 09:50