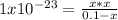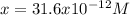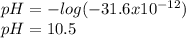Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
consider an exceptionally weak acid, HA, with Ka= 1 x 10-20. you make 0.1M solution of the salt NA....
Questions

Chemistry, 15.10.2020 20:01

Mathematics, 15.10.2020 20:01





Mathematics, 15.10.2020 20:01

Biology, 15.10.2020 20:01


Mathematics, 15.10.2020 20:01


English, 15.10.2020 20:01

English, 15.10.2020 20:01



Mathematics, 15.10.2020 20:01


Mathematics, 15.10.2020 20:01

Mathematics, 15.10.2020 20:01

Computers and Technology, 15.10.2020 20:01

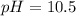
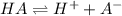
![Ka=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0682/3092/39962.png)
 due to the reaction extent is:
due to the reaction extent is: