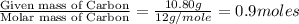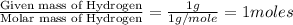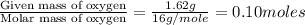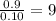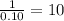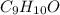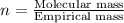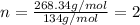Chemistry, 10.06.2020 19:57 calhountoiyonou0gjb
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 39.61 g CO2 and 9.01 g H2O. The molar mass of equilin is 268.34 g/mol. Find its molecular formula.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon,...
Questions


Mathematics, 27.04.2020 03:11

Chemistry, 27.04.2020 03:11

English, 27.04.2020 03:12



Business, 27.04.2020 03:12

Biology, 27.04.2020 03:12


Mathematics, 27.04.2020 03:12

Mathematics, 27.04.2020 03:12


Mathematics, 27.04.2020 03:12



English, 27.04.2020 03:12

Mathematics, 27.04.2020 03:12

Social Studies, 27.04.2020 03:12


Mathematics, 27.04.2020 03:12

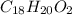
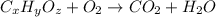
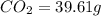
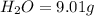
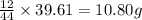 of carbon will be contained.
of carbon will be contained.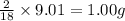 of hydrogen will be contained.
of hydrogen will be contained.