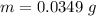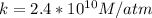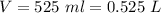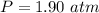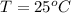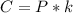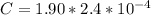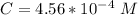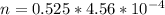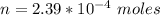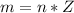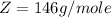Chemistry, 10.06.2020 19:57 jerseygirl3467
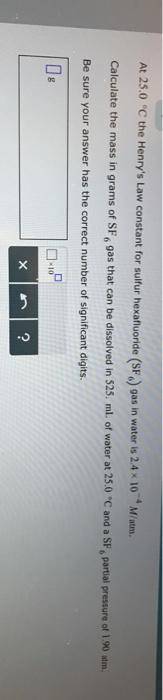
At 25.0 °C the Henry's Law constant for sulfur hexafluoride (SP) gas in water is 2.4x 10 M/atm Calculate the mass in grams of SFo, gas that can be dissolved in S25. ml. of water at 25.0 C and a SF, partial pressure of 1.90 atm Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
At 25.0 °C the Henry's Law constant for sulfur hexafluoride (SP) gas in water is 2.4x 10 M/atm Calcu...
Questions

History, 24.04.2020 00:59

Social Studies, 24.04.2020 00:59


Mathematics, 24.04.2020 00:59



History, 24.04.2020 00:59


History, 24.04.2020 00:59




Mathematics, 24.04.2020 00:59


History, 24.04.2020 00:59

History, 24.04.2020 01:00


Chemistry, 24.04.2020 01:00

Mathematics, 24.04.2020 01:00