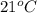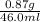Chemistry, 09.06.2020 14:57 brennae8529
A geochemist in the field takes a 46.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 21°C, and caps the sample carefully. Back in the lab, the geochemist filters the sample and then evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries and weighs the crystals. They weigh 0.87 g.
Required:
Using only the information above, can you calculate the solubility of X in water at 21°C? If yes, calculate it.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2



Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
A geochemist in the field takes a 46.0 mL sample of water from a rock pool lined with crystals of a...
Questions

Mathematics, 12.12.2020 18:00

Mathematics, 12.12.2020 18:00

Mathematics, 12.12.2020 18:00

Mathematics, 12.12.2020 18:00

Mathematics, 12.12.2020 18:00

English, 12.12.2020 18:10

Health, 12.12.2020 18:10

Mathematics, 12.12.2020 18:10


Mathematics, 12.12.2020 18:10

Health, 12.12.2020 18:10

Mathematics, 12.12.2020 18:10

SAT, 12.12.2020 18:10

Business, 12.12.2020 18:10



History, 12.12.2020 18:10

Mathematics, 12.12.2020 18:10


Mathematics, 12.12.2020 18:10

 g/ml.
g/ml.