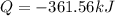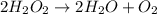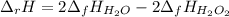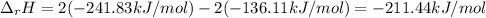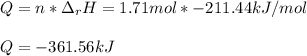Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3

Chemistry, 23.06.2019 17:30
Two examples of energy transformations are shown. the energy transformations are similar because they both involve transformations that begin with chemical energy. begin with electrical energy. result in radiant energy. result in mechanical energy.
Answers: 2
You know the right answer?
The decomposition of hydrogen peroxide, H2O2, has been used to provide thrust in the control jets of...
Questions

Mathematics, 28.08.2019 03:20



Mathematics, 28.08.2019 03:20





Mathematics, 28.08.2019 03:20





Social Studies, 28.08.2019 03:20



Computers and Technology, 28.08.2019 03:20



Computers and Technology, 28.08.2019 03:20