Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
A reaction mixture was found to contain 0.280 moles of HI (g), 2.09×10^-2 moles of H2 (g), and 4.14×10^-2 moles of I2 (g), in a 1.00 liter container.
Required:
a. Is the reaction at equilibrium?
b. What direction must it run in order to reach equilibrium?
c. The reaction
1. must run in the forward direction to reach equilibrium.
2. must run in the reverse direction to reach equilibrium.
3. is at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, and calculate its density. here is a summary of the steps required: add water by clicking and holding prepare a known volume of water button. until the desired volume of water has been added. if more than the desired volume is added, click the reset button. button and redo the procedure. a single click will add about 21.0 ml of water. to set the mass, click and hold weigh out metal button. until the desired amount of metal is added to the weighing pan. once the desired mass of the metal is added, release the button. transfer the metal to water and then click on calculate density button. to see how the density is calculated using water displacement to measure the volume of the solid. to save time you can approximate the initial volume of water to â±1 ml and the initial mass of the solid to â±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? check all that apply.
Answers: 1



Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
...
...
Questions

Social Studies, 28.04.2021 18:20

Chemistry, 28.04.2021 18:20


Mathematics, 28.04.2021 18:20



Mathematics, 28.04.2021 18:20

Mathematics, 28.04.2021 18:20

German, 28.04.2021 18:20

Mathematics, 28.04.2021 18:20







English, 28.04.2021 18:20



![Qc=\frac{[C]^{c}*[D]^{d} } {[A]^{a}*[B]^{b}}](/tpl/images/0680/9750/5af8a.png)
![Qc=\frac{[H_{2} ]*[I_{2} ] } {[HI]^{2}}](/tpl/images/0680/9750/8091f.png)
![[H_{2} ]=\frac{2.09*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/734fa.png) =2.09*10⁻²
=2.09*10⁻² 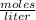
![[I_{2} ]=\frac{4.14*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/92c8b.png) =4.14*10⁻²
=4.14*10⁻² ![[I_{2} ]=\frac{0.280 moles}{1 Liter}](/tpl/images/0680/9750/e5237.png) = 0.280
= 0.280