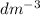Chemistry, 07.06.2020 07:57 bayleeharris8p78txa
What is the concentration of a solution with a volume of 660L that contains 33.4g of AlCO3?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Dying the folding patterns of protein molecules can microbiologists better understand cellular processes as well as some diseases, such as alzheimer’s, that are caused by proteins that have misfolded. the folding of these complicated molecules can be simulated on computers, but it takes a lot of processor power and time for even expensive supercomputers to do this. a group of researchers at stanford university developed software that can be used to distribute the processing of data to anyone who is willing to donate time on their idle personal computers. as a result, the researchers have been able to achieve protein-folding simulations that are far better than those other computing methods have done. which statement best describes the work of these researchers? the work is not scientific because the data are not processed in one location. the work is not scientific because the simulations are not reproducible. the researchers applied creativity to solve a problem in running an experiment. the researchers used only well-established scientific techniques.
Answers: 3

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1
You know the right answer?
What is the concentration of a solution with a volume of 660L that contains 33.4g of AlCO3?...
Questions


Mathematics, 21.09.2019 10:20

Geography, 21.09.2019 10:20

Biology, 21.09.2019 10:20

Social Studies, 21.09.2019 10:20

Mathematics, 21.09.2019 10:20


English, 21.09.2019 10:20



Mathematics, 21.09.2019 10:20

History, 21.09.2019 10:20

English, 21.09.2019 10:20

Mathematics, 21.09.2019 10:20

Mathematics, 21.09.2019 10:20


History, 21.09.2019 10:20

Biology, 21.09.2019 10:20

Mathematics, 21.09.2019 10:20

Social Studies, 21.09.2019 10:20

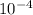 mol.
mol.