
Chemistry, 07.06.2020 05:02 Yungnaudie
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . What is the theoretical yield of sodium sulfate formed from the reaction of of sulfuric acid and of sodium hydroxide

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1
You know the right answer?
Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium sulfate and liqui...
Questions


English, 19.05.2020 02:21


Law, 19.05.2020 02:21

History, 19.05.2020 02:21

History, 19.05.2020 02:21


Mathematics, 19.05.2020 02:21

Mathematics, 19.05.2020 02:21




Mathematics, 19.05.2020 02:22

Mathematics, 19.05.2020 02:22

Mathematics, 19.05.2020 02:22

Mathematics, 19.05.2020 02:22

Social Studies, 19.05.2020 02:22



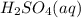 +
+ 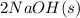 →
→ 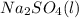 +
+ 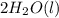
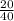 = 0.5 moles
= 0.5 moles

