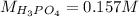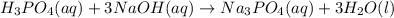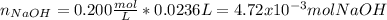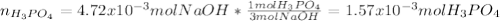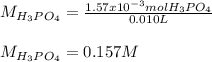Chemistry, 07.06.2020 04:57 isaiahst573
f 23.6 mL of 0.200 M NaOH is required to neutralize 10.00 mL of a H3PO4 solution , what is the concentration of the phosphoric acid solution?Start by balancing the equation for the reaction: H3PO4(aq) + NaOH(aq) → Na3PO4(aq) + H2O(l)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
f 23.6 mL of 0.200 M NaOH is required to neutralize 10.00 mL of a H3PO4 solution , what is the conce...
Questions


Mathematics, 25.09.2019 09:30

Chemistry, 25.09.2019 09:30


History, 25.09.2019 09:30


Mathematics, 25.09.2019 09:30









Mathematics, 25.09.2019 09:30


Biology, 25.09.2019 09:30

Mathematics, 25.09.2019 09:30

Mathematics, 25.09.2019 09:30