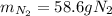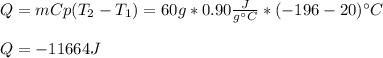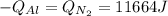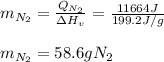Chemistry, 07.06.2020 04:58 isabella4141
A 60 g piece of aluminum at 20°C is cooled to -196°C by placing it in a large container of liquid nitrogen at that temperature. How much nitrogen is vaporized? (Assume that the specific heat of aluminum is constant and is equal to 0.90 kJ/kg·K and that the vaporized nitrogen's temperature does not change.)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
A 60 g piece of aluminum at 20°C is cooled to -196°C by placing it in a large container of liquid ni...
Questions

Mathematics, 02.11.2020 23:00


Mathematics, 02.11.2020 23:00



English, 02.11.2020 23:00


English, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00




Health, 02.11.2020 23:00


Mathematics, 02.11.2020 23:00

Mathematics, 02.11.2020 23:00