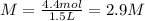Chemistry, 07.06.2020 03:00 mazielynn84
What is the molarity of a solution that has 4.4 moles BaCl2 in 1.5 Liters of solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
What is the molarity of a solution that has 4.4 moles BaCl2 in 1.5 Liters of solution?...
Questions

Computers and Technology, 25.11.2020 01:00

English, 25.11.2020 01:00

Physics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00



Computers and Technology, 25.11.2020 01:00




Mathematics, 25.11.2020 01:00



Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00


Mathematics, 25.11.2020 01:00

Mathematics, 25.11.2020 01:00

Chemistry, 25.11.2020 01:00