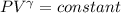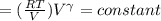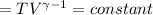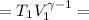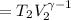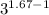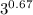Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

You know the right answer?
Calculate the final temperature of 12.0 g of Argon (considered as an ideal gas) that is expanded rev...
Questions

Mathematics, 15.04.2021 04:00

Mathematics, 15.04.2021 04:00

Mathematics, 15.04.2021 04:00

Mathematics, 15.04.2021 04:00

Business, 15.04.2021 04:00




Mathematics, 15.04.2021 04:00

Mathematics, 15.04.2021 04:00

Mathematics, 15.04.2021 04:10




Mathematics, 15.04.2021 04:10


Arts, 15.04.2021 04:10


Computers and Technology, 15.04.2021 04:10