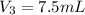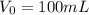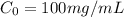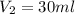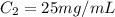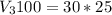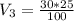Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
You have a 100 ml stock solution of 100 mg/ml ampicillin in deionized water. You want to make 30 ml...
Questions





Mathematics, 12.04.2021 22:30



Biology, 12.04.2021 22:30

Social Studies, 12.04.2021 22:30


Mathematics, 12.04.2021 22:30


History, 12.04.2021 22:30

Physics, 12.04.2021 22:30



Mathematics, 12.04.2021 22:30


History, 12.04.2021 22:30