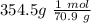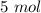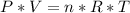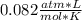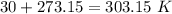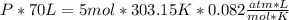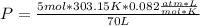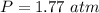Chemistry, 06.06.2020 04:01 khenalilovespandas
354.5 g of chlorine gas (MW = 70.9 g/mol) is held in a vessel with a fixed volume of 70. L.
What is the pressure of the gas in atmospheres if it's temperature is 30.0°C?
___ atm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
354.5 g of chlorine gas (MW = 70.9 g/mol) is held in a vessel with a fixed volume of 70. L.
What is...
Questions

Social Studies, 18.02.2021 16:20

Mathematics, 18.02.2021 16:20


Advanced Placement (AP), 18.02.2021 16:20


Social Studies, 18.02.2021 16:20

Mathematics, 18.02.2021 16:20

English, 18.02.2021 16:20



Mathematics, 18.02.2021 16:20



Physics, 18.02.2021 16:20

History, 18.02.2021 16:20





Mathematics, 18.02.2021 16:20