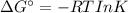Chemistry, 06.06.2020 03:57 cooljazzy1234
43. Calculate the equilibrium constant at the temperature given. (a) O2 (g) + 2F2 (g) ⟶ 2F2 O(g) (T = 100 °C) (b) I2 (s) + Br2 (l) ⟶ 2IBr(g) (T = 0.0 °C) (c) 2LiOH(s) + CO2 (g) ⟶ Li2CO3 (s) + H2 O(g) (T = 575 °C) (d) N2 O3 (g) ⟶ NO(g) + NO2 (g) (T = −10.0 °C) (e) SnCl4 (l) ⟶ SnCl4 (g) (T = 200 °C)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
You know the right answer?
43. Calculate the equilibrium constant at the temperature given. (a) O2 (g) + 2F2 (g) ⟶ 2F2 O(g) (T...
Questions



Health, 04.07.2019 11:30



Mathematics, 04.07.2019 11:30

Mathematics, 04.07.2019 11:30



Mathematics, 04.07.2019 11:30




Mathematics, 04.07.2019 11:30






Health, 04.07.2019 11:30

![\Delta G_f[F_2O]=41.9kJ/mol =41900J/mol](/tpl/images/0678/2670/ad240.png)
![\Delta G_f[O_2]=0\\\\ \Delta G_f[F_2]=0](/tpl/images/0678/2670/20ae9.png)
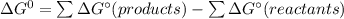
![\Delta G^\circ = [2 \times 41900]-0\\\\=83800J/mol](/tpl/images/0678/2670/d8ac8.png)