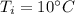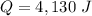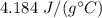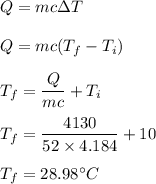Chemistry, 06.06.2020 04:00 zaniyastubbs9
A 52-gram sample of water that has an initial temperature of 10.0 °C absorbs 4,130 joules. If the specific heat of water is 4.184 J/(g °C), what is the final temperature of the water?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1
You know the right answer?
A 52-gram sample of water that has an initial temperature of 10.0 °C absorbs 4,130 joules. If the sp...
Questions

Mathematics, 04.03.2021 07:10

History, 04.03.2021 07:10

History, 04.03.2021 07:10


Mathematics, 04.03.2021 07:10

Chemistry, 04.03.2021 07:10



History, 04.03.2021 07:10

Mathematics, 04.03.2021 07:10

Mathematics, 04.03.2021 07:10


Mathematics, 04.03.2021 07:10


Mathematics, 04.03.2021 07:10

Computers and Technology, 04.03.2021 07:10

Social Studies, 04.03.2021 07:10



Mathematics, 04.03.2021 07:20