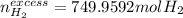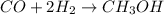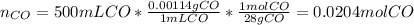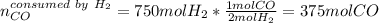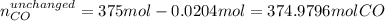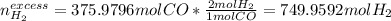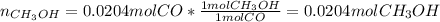Chemistry, 05.06.2020 16:00 nxusasmangaliso1191
Methanol CH3OH is the simplest of the alcohols . It is sinthesized by the reaction of hydrogen and carbon monoxide.
CO + H2 > CH3OH
a) If 500 ml CO and 750 mol H2 are present, which is the limiting reactant?
b) How many mols of excess reactant remain unchange?
c) How many moles of CH3OH are formed ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
Methanol CH3OH is the simplest of the alcohols . It is sinthesized by the reaction of hydrogen and c...
Questions

History, 10.09.2021 20:50

Mathematics, 10.09.2021 20:50

Mathematics, 10.09.2021 20:50

Mathematics, 10.09.2021 20:50

Chemistry, 10.09.2021 20:50

Mathematics, 10.09.2021 20:50

Biology, 10.09.2021 20:50


Chemistry, 10.09.2021 20:50





Mathematics, 10.09.2021 20:50


Mathematics, 10.09.2021 20:50




History, 10.09.2021 20:50