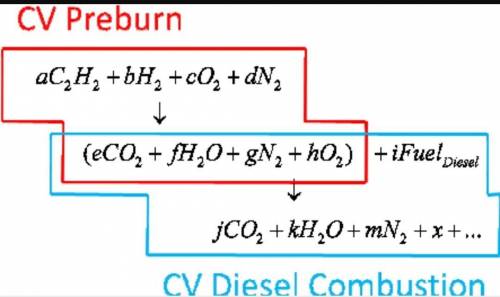
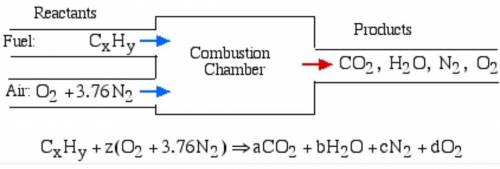
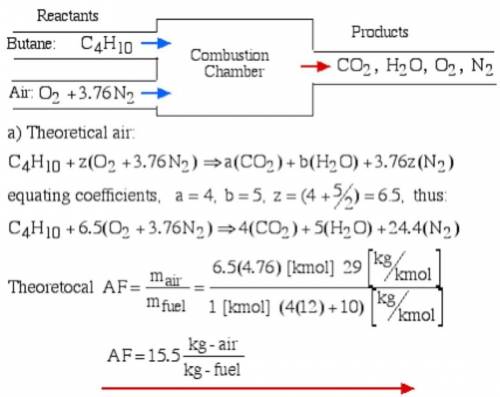
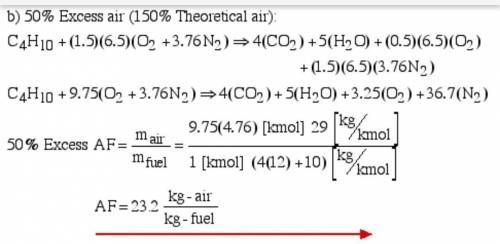
6.) A hydrocarbon molecule of
formula CxHy is completely
burnt in excess oxygen. The
comb...


Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

You know the right answer?
Questions

Advanced Placement (AP), 02.06.2021 08:00

English, 02.06.2021 08:00


Mathematics, 02.06.2021 08:00

Mathematics, 02.06.2021 08:00

History, 02.06.2021 08:00


Mathematics, 02.06.2021 08:00


Mathematics, 02.06.2021 08:00

Mathematics, 02.06.2021 08:00


Spanish, 02.06.2021 08:00

English, 02.06.2021 08:00

Mathematics, 02.06.2021 08:00

Mathematics, 02.06.2021 08:00

Mathematics, 02.06.2021 08:00

English, 02.06.2021 08:00