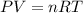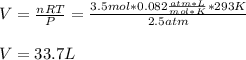Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

You know the right answer?
3.5 moles of argon gas are maintained at a temperature of 293K and a pressure of 2.5 atm. What is th...
Questions


Mathematics, 26.04.2020 15:57




Arts, 26.04.2020 15:57

Mathematics, 26.04.2020 15:58


Mathematics, 26.04.2020 15:58

Mathematics, 26.04.2020 15:58

Chemistry, 26.04.2020 15:58




Geography, 26.04.2020 15:59


Physics, 26.04.2020 16:01

Mathematics, 26.04.2020 16:02