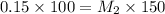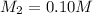Chemistry, 04.06.2020 17:58 swkgp3cevk
If I add water to 100mL of a 0.15 M NaOH solution until the final volume is 150mL, what will the molarity of the diluted solution be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
If I add water to 100mL of a 0.15 M NaOH solution until the final volume is 150mL, what will the mol...
Questions



Chemistry, 18.12.2020 02:50

Advanced Placement (AP), 18.12.2020 02:50




Spanish, 18.12.2020 02:50





Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50

Spanish, 18.12.2020 02:50


Mathematics, 18.12.2020 02:50

Mathematics, 18.12.2020 02:50


Mathematics, 18.12.2020 02:50

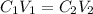
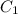 = concentration of concentrated solution = 0.15 M
= concentration of concentrated solution = 0.15 M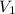 = volume of concentrated solution = 100 ml
= volume of concentrated solution = 100 ml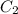 = concentration of diluted solution= ?
= concentration of diluted solution= ?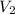 = volume of diluted solution= 150 ml
= volume of diluted solution= 150 ml