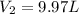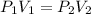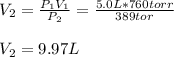Chemistry, 03.06.2020 08:57 ejuarez2020
A balloon with a volume of 5.0 L is filled with a gas at 760 tore. If the pressure is reduced to 389 torr without a change in temperature. What will be the volume of the balloon?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
A balloon with a volume of 5.0 L is filled with a gas at 760 tore. If the pressure is reduced to 389...
Questions



Mathematics, 16.10.2020 18:01

History, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01


Medicine, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01




Mathematics, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01