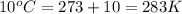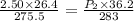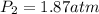7. A 26.4-ml sample of ethylene gas, C2H4, has a pres-sure of 2.50 atm at 2.5°C. If the
volume...

Chemistry, 03.06.2020 07:57 Crtive6538
7. A 26.4-ml sample of ethylene gas, C2H4, has a pres-sure of 2.50 atm at 2.5°C. If the
volume is increased to 36.2 mL and the temperature is raised to 10°C, what is the
new pressure. (Hint: Three variables have been given so what equation will you
use?)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
Questions


English, 04.05.2021 17:10


History, 04.05.2021 17:10




Mathematics, 04.05.2021 17:10

Mathematics, 04.05.2021 17:10



Physics, 04.05.2021 17:10


Mathematics, 04.05.2021 17:10

Mathematics, 04.05.2021 17:10

English, 04.05.2021 17:10



English, 04.05.2021 17:10

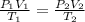
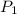 = initial pressure of gas = 2.50 atm
= initial pressure of gas = 2.50 atm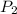 = final pressure of gas = ?
= final pressure of gas = ?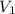 = initial volume of gas = 26.4 ml
= initial volume of gas = 26.4 ml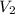 = final volume of gas = 36.2 ml
= final volume of gas = 36.2 ml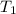 = initial temperature of gas =
= initial temperature of gas = 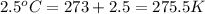
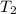 = final temperature of gas =
= final temperature of gas =