Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
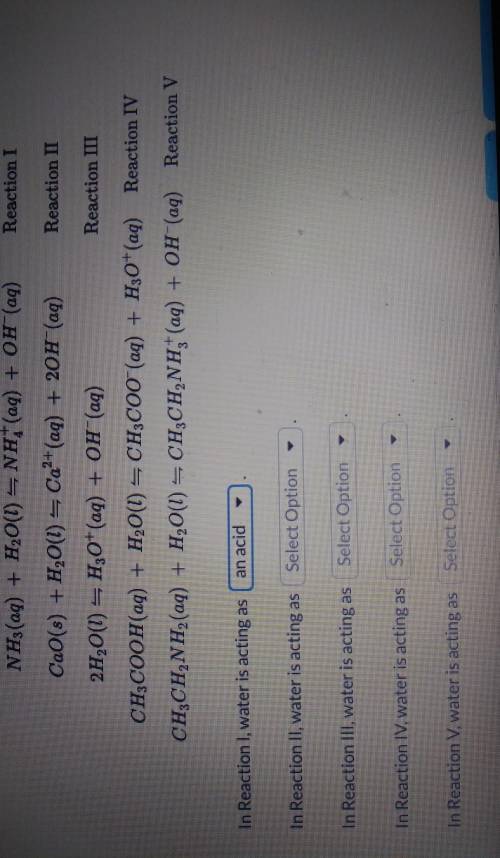
For each of the reactions below, determine whether water is acting as a Brønsted-Lowry acid, a Brøns...
Questions



Social Studies, 02.06.2020 19:58




Mathematics, 02.06.2020 19:58



History, 02.06.2020 19:58

History, 02.06.2020 19:58

Mathematics, 02.06.2020 19:58







Computers and Technology, 02.06.2020 19:58

Mathematics, 02.06.2020 19:58