N2(g) + 3H2(g) <-> 2NH3(g) + energy

Chemistry, 02.06.2020 19:58 roxymiller3942
Given the equation for a system at equilibrium:
N2(g) + 3H2(g) <-> 2NH3(g) + energy
If only the concentration of N2(g) is increased, the concentration of
1. NH3(g) increases
2. NH3(g) remains the same
3. H2(g) increases
4. H2(g) remains the same

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

You know the right answer?
Given the equation for a system at equilibrium:
N2(g) + 3H2(g) <-> 2NH3(g) + energy
N2(g) + 3H2(g) <-> 2NH3(g) + energy
Questions

Mathematics, 19.02.2021 20:10


Mathematics, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Chemistry, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Social Studies, 19.02.2021 20:10

Social Studies, 19.02.2021 20:10


Mathematics, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10

Social Studies, 19.02.2021 20:10

Mathematics, 19.02.2021 20:10


Mathematics, 19.02.2021 20:10


Biology, 19.02.2021 20:10

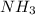 increases.
increases.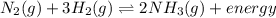
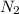 is increased , according to the Le-Chatlier's principle, the reaction has to shift to right or forward direction. In order to do that the concentration of products has to increase.
is increased , according to the Le-Chatlier's principle, the reaction has to shift to right or forward direction. In order to do that the concentration of products has to increase.

