
Chemistry, 01.06.2020 15:58 babygirl091502
What is the molarity of a solution formed by dissolving 100 g of Ca(No3)2 and 450 mL of solution
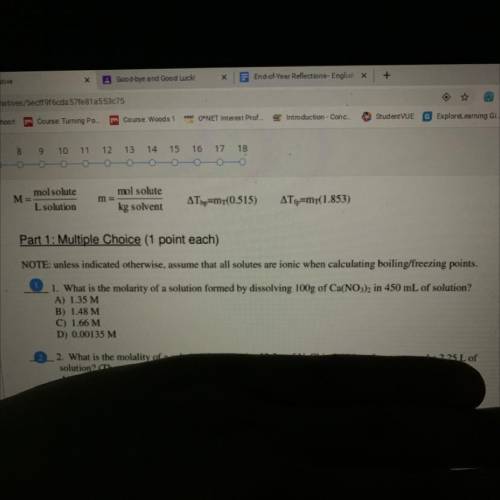

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
What is the molarity of a solution formed by dissolving 100 g of Ca(No3)2 and 450 mL of solution
Questions

Biology, 09.07.2019 02:00

Mathematics, 09.07.2019 02:00




Arts, 09.07.2019 02:00

Biology, 09.07.2019 02:00

Chemistry, 09.07.2019 02:00

Chemistry, 09.07.2019 02:00

Chemistry, 09.07.2019 02:00




Mathematics, 09.07.2019 02:00






Mathematics, 09.07.2019 02:00



