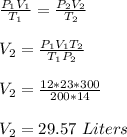Chemistry, 01.06.2020 12:57 potato3999
If I initially have a gas at a pressure of 12 atm, a volume of 23 Liters and a temperature of 200K, and then I raise the pressure to 14 atm and increase the temperature to 300K, what is the new volume?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1


Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

You know the right answer?
If I initially have a gas at a pressure of 12 atm, a volume of 23 Liters and a temperature of 200K,...
Questions

Mathematics, 30.04.2021 09:50

Mathematics, 30.04.2021 09:50



History, 30.04.2021 09:50

Mathematics, 30.04.2021 09:50



Physics, 30.04.2021 09:50


English, 30.04.2021 09:50

Social Studies, 30.04.2021 14:00


Physics, 30.04.2021 14:00


Chemistry, 30.04.2021 14:00


Mathematics, 30.04.2021 14:00


English, 30.04.2021 14:00