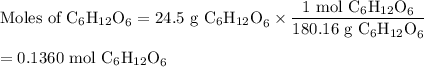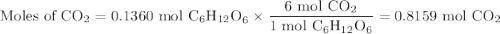Chemistry, 01.06.2020 04:58 maxicanofb0011
The metabolic oxidation of glucose, C6H12O6, in our bodies produces CO2, which is expelled from our lungs as a gas.
C6H12O6(aq) + 6 O2(g) → 6 CO2(g) + 6 H2O(l)
Calculate the volume of dry CO2 produced at body temperature (37°C) and 0.960 atm when 24.5 g of glucose is consumed in this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1

Chemistry, 23.06.2019 16:30
Amodel of an atom is shown below. which element is represented by this model of an atom? boron, carbon, neon, or sodium?
Answers: 1
You know the right answer?
The metabolic oxidation of glucose, C6H12O6, in our bodies produces CO2, which is expelled from our...
Questions



Mathematics, 05.03.2021 03:40


Arts, 05.03.2021 03:40

Mathematics, 05.03.2021 03:40

Mathematics, 05.03.2021 03:40

History, 05.03.2021 03:40





Mathematics, 05.03.2021 03:40

Mathematics, 05.03.2021 03:40


Physics, 05.03.2021 03:40

Mathematics, 05.03.2021 03:40