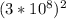Chemistry, 31.05.2020 00:57 amusgrave9175
Use the nuclear decay reaction in the picture to answer the following question.
i. How does the reaction demonstrate the laws of conservation of charge and conservation of nucleon number?
ii. What is the binding energy of one mole of atomic mass: 234, number of proton: 90, Thorium if the mass defect is 1.908 g/mol?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
Use the nuclear decay reaction in the picture to answer the following question.
i. How do...
i. How do...
Questions

Mathematics, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40


Health, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40

Mathematics, 03.05.2021 03:40



Mathematics, 03.05.2021 03:40

Physics, 03.05.2021 03:40


Mathematics, 03.05.2021 03:50

History, 03.05.2021 03:50


Mathematics, 03.05.2021 03:50

Computers and Technology, 03.05.2021 03:50



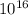 J.
J.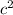
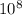 . So that:
. So that: