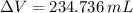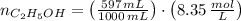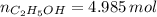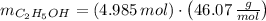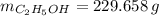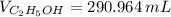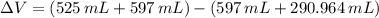A solution is prepared by mixing 525 mL of ethanol with 597 mL of water. The molarity of ethanol in the resulting solution is 8.35 M. The density of ethanol at this temperature is 0.7893 g/mL. Calculate the difference in volume between the total volume of water and ethanol that were mixed to prepare the solution and the actual volume of the solution. g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2


Chemistry, 23.06.2019 19:30
The following data was collected when a reaction was performed experimentally in the laboratory ; determine the maximum amount of alcl3 that was produced during the experiment explain how you determined this reactants products al(no3)3 nacl. nano3 alcl3 4 moles 9 moles ? ?
Answers: 3

You know the right answer?
A solution is prepared by mixing 525 mL of ethanol with 597 mL of water. The molarity of ethanol in...
Questions


Mathematics, 04.03.2021 20:00


Mathematics, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00


Biology, 04.03.2021 20:00



Mathematics, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00

History, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00


Mathematics, 04.03.2021 20:00



Arts, 04.03.2021 20:00

Geography, 04.03.2021 20:00