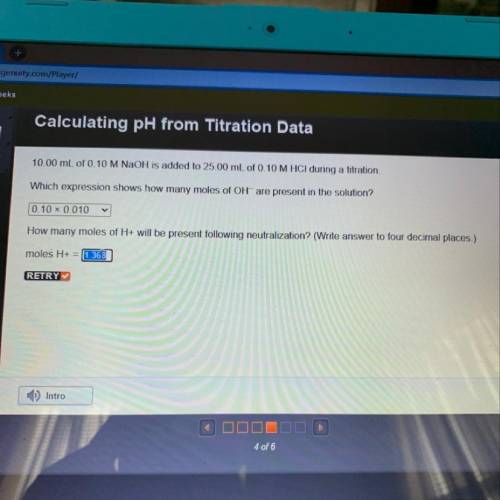
10.00 mL of 0.10 M NaOH is added to 25.00 mL of 0.10 M HCl during a titration,
Which expressio...

10.00 mL of 0.10 M NaOH is added to 25.00 mL of 0.10 M HCl during a titration,
Which expression shows how many moles of OH- are present in the solution?
How many moles of H+ will be present following neutralization? (Write answer to four decimal places.)
moles H+ =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Questions


Mathematics, 18.10.2020 15:01



Mathematics, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01


English, 18.10.2020 15:01




Chemistry, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01





English, 18.10.2020 15:01

English, 18.10.2020 15:01



