

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
What is the pH of 1.0 L of 0.20 M aqueous solution of ammonium chloride () to which 150 ml of a 0.50...
Questions

History, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00

History, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00



Mathematics, 10.03.2021 22:00



Mathematics, 10.03.2021 22:00

English, 10.03.2021 22:00

Mathematics, 10.03.2021 22:00


History, 10.03.2021 22:00


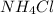 ) to which 150 ml of a 0.50 M aqueous NaOH solution is added? Assuming that
) to which 150 ml of a 0.50 M aqueous NaOH solution is added? Assuming that 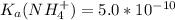 in this exercise.
in this exercise.

