
Chemistry, 30.05.2020 09:57 liamgreene90
If you start with 6 mol of nitrogen gas (N2+) what mass (g) of ammonia (NH4) will be produced?
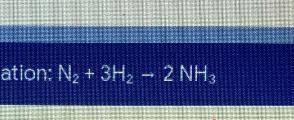

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
If you start with 6 mol of nitrogen gas (N2+) what mass (g) of ammonia (NH4) will be produced?
Questions

Mathematics, 27.08.2019 16:30



Health, 27.08.2019 16:30






History, 27.08.2019 16:30

Mathematics, 27.08.2019 16:30


Chemistry, 27.08.2019 16:30


English, 27.08.2019 16:30

Spanish, 27.08.2019 16:30

Spanish, 27.08.2019 16:30



Mathematics, 27.08.2019 16:30



