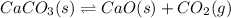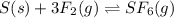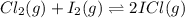Chemistry, 29.05.2020 13:57 lanakay2006
Changes in pressure can have a large effect on equilibrium systems containing gaseous components.
1. changing the concentration of gaseous components
2. adding an inert gas has no effect since the gas does not take part in the reaction, all partial pressures stay the same
3.changing the volume of the reaction vessel. This will cause a shift in the equilibrium position if the number of moles of gas is different on the reactant and product side (so Δn = n products - n reactants)
How would you change the volume for each of the following reactions to increase the yield of the product(s)?1. CaCO3(s) ⇋ CaO(s) + CO2(g) (increase, decrease, no change)2. S(s) + 3F2(g) ⇋ SF6(g) (increase, decrease, no change)3. Cl2(g) + I2(g) ⇋ 2ICl(g) (increase, decrease, no change)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
Changes in pressure can have a large effect on equilibrium systems containing gaseous components.
Questions


History, 28.05.2020 21:04


English, 28.05.2020 21:04

Chemistry, 28.05.2020 21:04

Mathematics, 28.05.2020 21:04

History, 28.05.2020 21:04

Mathematics, 28.05.2020 21:04

Arts, 28.05.2020 21:04

English, 28.05.2020 21:04


Mathematics, 28.05.2020 21:04


Mathematics, 28.05.2020 21:04

Computers and Technology, 28.05.2020 21:04



Chemistry, 28.05.2020 21:05

Mathematics, 28.05.2020 21:05