
Chemistry, 29.05.2020 22:58 yulimariu27
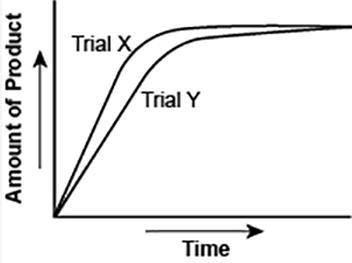
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A) Trial X, because the final volume of product formed is lower than Trial Y.
B) Trial X, because this reaction was initially fast and later stopped completely.
C) Trial Y, because the reaction was initially slow and later stopped completely.
D) Trial Y, because the volume of product formed per unit time is lower than Trial X.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions


Mathematics, 04.12.2021 02:10

History, 04.12.2021 02:10


Mathematics, 04.12.2021 02:10







Mathematics, 04.12.2021 02:10

English, 04.12.2021 02:10

World Languages, 04.12.2021 02:10



Mathematics, 04.12.2021 02:10

Mathematics, 04.12.2021 02:10




