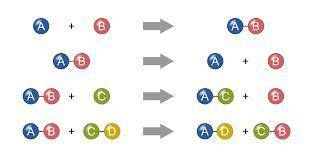
I NEED HELP PLEASE, THANKS!
Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double displacement reaction with aqueous sodium chloride, NaCl, in which a precipitate forms. If the precipitate contains lead, write the balanced chemical equation for this reaction. Be sure to include the states of each of the reactants and products.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
I NEED HELP PLEASE, THANKS!
Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double displ...
Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double displ...
Questions

Mathematics, 14.08.2020 21:01


Chemistry, 14.08.2020 21:01








History, 14.08.2020 21:01



Mathematics, 14.08.2020 21:01



Mathematics, 14.08.2020 21:01



Computers and Technology, 14.08.2020 21:01