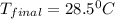Chemistry, 29.05.2020 20:00 rawaanasser12245
The specific heat of mercury is 0.138 J/g Co . If 452g of mercury at 85.0 Co are placed in 145g of water at 23.0 Co , what will be the final temperature for both the mercury and the water?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
The specific heat of mercury is 0.138 J/g Co . If 452g of mercury at 85.0 Co are placed in 145g of w...
Questions

English, 21.05.2020 04:10

English, 21.05.2020 04:10




History, 21.05.2020 04:10


Mathematics, 21.05.2020 04:10

Law, 21.05.2020 04:10



English, 21.05.2020 04:10

History, 21.05.2020 04:11

History, 21.05.2020 04:11

English, 21.05.2020 04:11

Computers and Technology, 21.05.2020 04:11

Mathematics, 21.05.2020 04:11



Physics, 21.05.2020 04:11

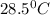
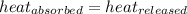
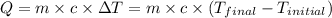
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0670/5209/09236.png) .................(1)
.................(1)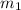 = mass of mercury = 425 g
= mass of mercury = 425 g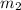 = mass of water = 145 g
= mass of water = 145 g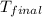 = final temperature = ?
= final temperature = ?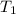 = temperature of mercury =
= temperature of mercury = 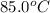
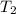 = temperature of water =
= temperature of water = 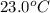
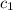 = specific heat of mercury =
= specific heat of mercury = 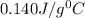
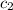 = specific heat of water=
= specific heat of water= 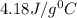
![-425\times 0.140\times (T_{final}-85.0)=[145\times 4.184\times (T_{final}-23.0)]](/tpl/images/0670/5209/c9617.png)